Effect of time on Ni and Cd leaching recovery (temperature=45 °C, NH4OH... | Download Scientific Diagram

Alkaline leaching of nickel bearing ammonium jarosite precipitate using KOH, NaOH and NH4OH in the presence of EDTA and Na2S | Semantic Scholar
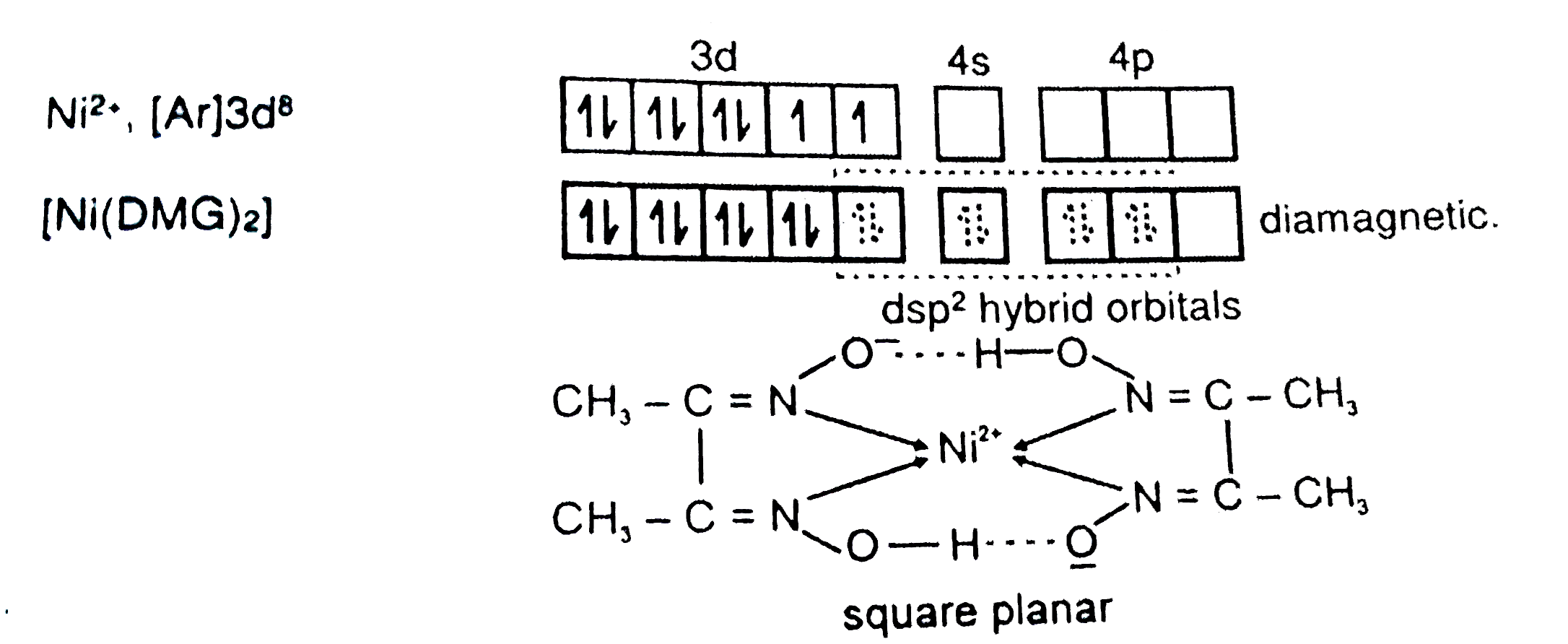
29. NiCl2 + NH4OH + dimethylglyoxime ——> A ( complex ) Incorrect statement for complex A is /are 1 Coordination number of metal ion is 4 2 Two five membered and two
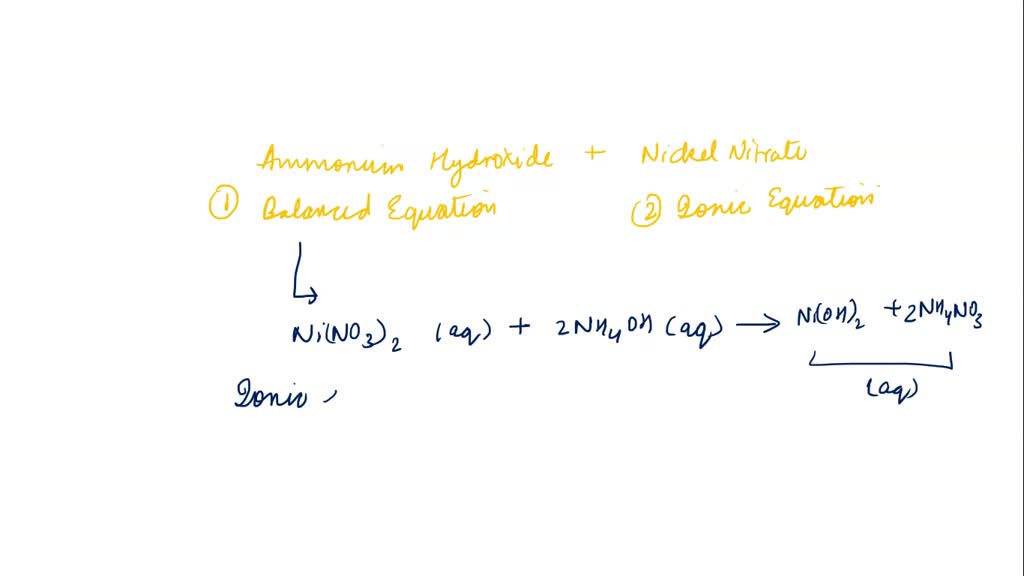
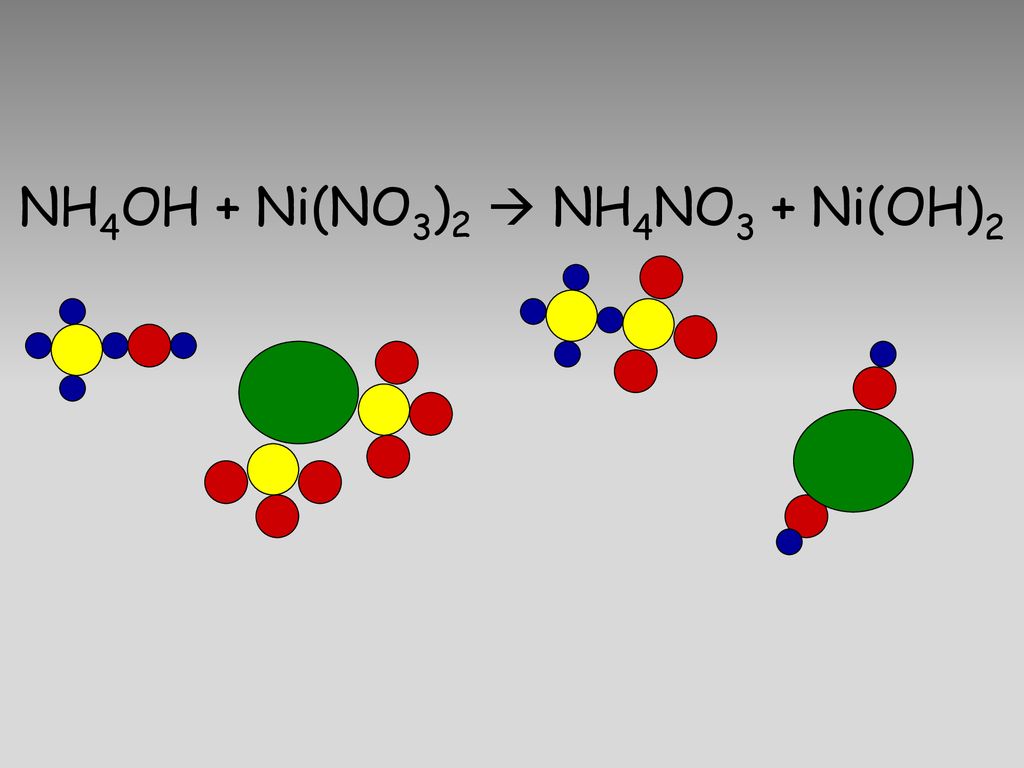
SOLVED: When an aqueous solution of NH4OH is mixed with an aqueous solution of Ni(NO3)2, a pale yellow precipitate forms. Write a balanced molecular equation for this reaction. Write the complete ionic

SOLVED: A nickel coordination compound, Ni(NH3)6Cl2, can be prepared by a procedure similar to the one you will use to prepare Cu(NH3)4SO4·H2O. The reaction can be represented stoichiometrically as NiCl2·6H2O + 6

Separation of Ni, Co, and Mn from Spent LiNi0.5Mn0.3Co0.2O2 Cathode Materials by Ammonia Dissolution | ACS Sustainable Chemistry & Engineering

Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect

NiCl_2 in the presence of dimethyl glycoxime (DMG) gives a complex which precipitates in the presence of NH_4OH, giving a bright red color.Draw its structure and show H bonding.

Epitaxial N–H-doped Ni1−x O and Ni2O3 with special planar defects by pulsed laser ablation of metallic Ni in aqueous ammonia | Applied Physics A
What is nh3 aqueous solution?. NH3 aqueous means aqueous solution of… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium










/fusion-NH4OH-generation-system-process-flow-diagram.png?width=710&name=fusion-NH4OH-generation-system-process-flow-diagram.png)